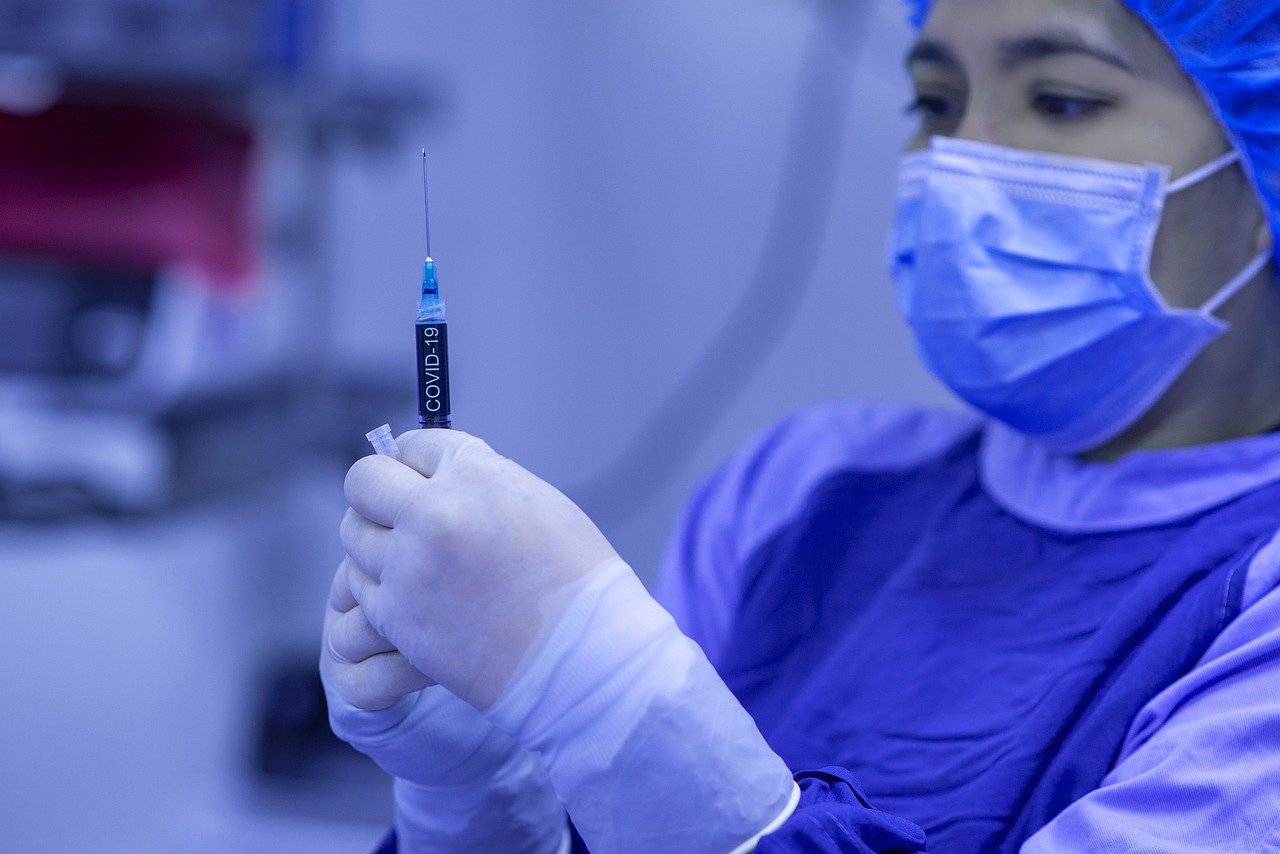
Concerns are growing over the safety and efficacy of a Covid-19 vaccine, it has been reported.
According to an article published in the scientific journal Nature, researchers have warned that vaccines could “stumble on safety trials, be fast-tracked because of politics or fail to meet the public’s expectations”.
Several scientists have expressed concerns about whether the vaccines will clear safety trials, what they will achieve if they do and the risk that the approval process will be influenced by politics.
The concerns were raised after vaccine manufacturers announced game-changing results from their vaccine trials.
Sponsors behind the vaccine have so far refused to release details about why vaccine trials were paused and restarted. Pfizer in particular, have refused to provide details on when the full vaccine trial data will be published.
AstraZeneca and Oxford both failed to respond to questions about calls for greater transparency.
This has only served to fuel concerns that political meddling could see a vaccine approved for emergency use without sufficient evidence that it works.
Raina MacIntyre, an epidemiologist at the University of New South Wales in Australia is one of the scientists quoted in the journal. She had expressed concerns after a participant in the vaccine trial developed symptoms of transverse myelitis. Transverse myelitis is an inflammation of both sides of one section of the spinal cord. Symptoms can include severe pain and discomfort, bladder problems, weakness in the arms and legs and severe physical disabilities. AstraZeneca says the person was later diagnosed with multiple sclerosis.
While there is only one reported case of a patient developing such symptoms, MacIntyre said that if it turns out that two people have developed transverse myelitis, given the relatively small number of people who have received the vaccine, that is notable.
She was quoted in Nature as saying: “If there’s another case, it’s going to be very hard for this trial to recover from that.”
MacIntyre notes that viral infections have been linked to both transverse myelitis and multiple sclerosis. Cases of transverse myelitis have also been observed in people with COVID-19.
In order to definitively rule out a link between the vaccine and the condition, researchers must run statistical analyses that compare rates of the conditions in participants who received the vaccines with those in people who got the placebo.
However, MacIntyre is not the only scientist to express concern.
Hilda Bastian, studies evidence-based medicine at Bond University in the Gold Coast, Australia. She said that the lack of transparency from the trial sponsors is a concern and could lead some participants to pull out, or cause people to decide not to get a vaccine once they are approved.
According to the research published in the journal, those expressing concern about the vaccines are not just the usual conspiracy theorists nor are they the ‘tinfoil hat’ crew.
Even researchers involved in vaccine design and testing have expressed reservations because of the potential that the approval process could be swayed by political, not just scientific, considerations.
These sentiments were expressed by Kurt Viele, director of modelling and simulation at Berry Consultants, which advises on clinical-trial designs, in Lexington, Kentucky.
He said: “I’m going to be looking at the safety data before I put a shot in my kids’ arms.”
Yannis Natsis, a policy manager at the advocacy group European Public Health Alliance in Brussels was also hesitant about the efficacy of the vaccine. He explained that while he is keen not to “give vaccines a bad name”, the lack of transparency continues to be a concern.
In the United States, a pathway for fast-tracking urgently needed treatments — an FDA Emergency Use Authorization (EUA) — has been part of the concern. The EUA sidesteps the usual drug-approval process and allows treatments to be used if they ‘may be effective’.
Herschel Nachlis, who studies health policy at Dartmouth College in Hanover, New Hampshire, said: “In being vague and non-transparent, it’s potentially susceptible to the appearance of political influence.”
Pfizer has so far failed to respond to questions about the rationale behind its clinical-trial design, or whether it plans to continue safety monitoring if a trial is stopped early.
Thomas Lumley, a biostatistician at the University of Auckland in New Zealand said that more time is needed to establish whether a vaccine reduces incidence of severe COVID-19.
Vaccine manufacturers are aiming for the vaccines to stop at least 50% of vaccinated people getting symptomatic COVID-19, the definition of success in the FDA guideline.
However, they are hoping for an efficacy of 60% or greater. The problem is that even 60% would not be enough to reach herd immunity, in which enough of the population has vaccine-derived immunity to stop the disease spreading.
Akashic Times is the UK’s only online, fully independent not-for-profit newspaper that brings you real news from across the globe.
If you want to keep ahead of what is really going on in the world, subscribe to our newspaper via the subscribe button and join our Facebook & Twitter pages. Subscription is completely free ofcourse















Follow Us!